Stereochemistry: Chirality underpins how molecules interact in biological assays, and therefore chiral purity in research compounds directly affects reproducibility, selectivity, and interpretability of results. As Pure Potion explains, even small enantiomeric impurities can change binding profiles or assay readouts; consequently, labs should treat stereochemical integrity as a critical QC parameter. All compounds discussed here are for research use only and are not intended for human or animal administration.
Why stereochemistry matters
Stereochemistry determines three-dimensional shape, and thus how a ligand fits into an active site. Moreover, enantiomers often display different physicochemical properties — for example, solubility, stability, and chromatographic behavior — which in turn influence experimental outcomes. Therefore, when researchers report activity data, they must document whether materials are single enantiomers, racemates, or enriched mixtures. Without such documentation, downstream comparisons across papers or labs become unreliable.
Analytical approaches to measure chiral purity
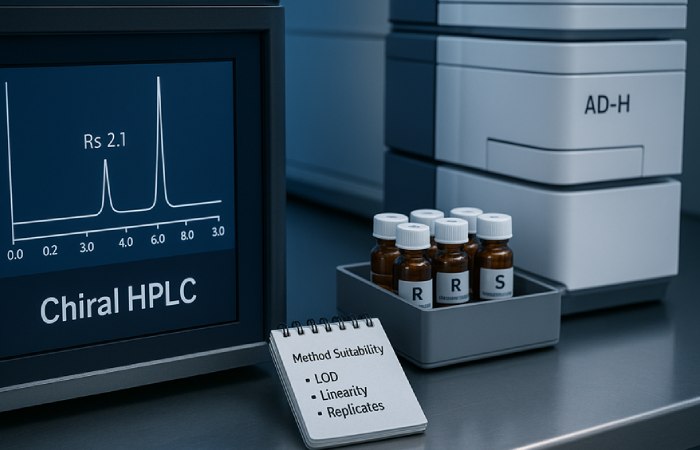
There are practical analytical routes to quantify enantiomeric excess and absolute configuration. Common methods include chiral HPLC with specific stationary phases, supercritical fluid chromatography (SFC), and chiral GC for volatile derivatives. Additionally, circular dichroism (CD) and nuclear magnetic resonance (NMR) with chiral shift reagents can provide orthogonal confirmation of stereochemistry. For robust reporting, use at least two orthogonal techniques; otherwise, minor impurities can evade detection and mislead downstream interpretation.
Advanced chiral separation techniques
When conventional chiral columns fail, more advanced workflows can help. For instance, preparative chiral chromatography allows isolation of pure enantiomers for standards. Alternatively, derivatization to diastereomers followed by achiral separation sometimes provides clearer quantitation. Furthermore, modern mass-spectrometry-coupled techniques offer high sensitivity for trace enantiomer detection. In practice, combining methods reduces false negatives and strengthens the claim of stereochemical purity.
Method validation and reporting standards
First, validate the analytical method across the expected concentration range. Second, evaluate resolution, limit of detection, and linearity for both enantiomers. Third, include system suitability criteria such as resolution (Rs) > 1.5 and reproducibility across replicates. Finally, document sample handling and storage conditions because temperature and light can induce racemization in susceptible compounds. Modern Aminos, for instance, routinely supplies COAs that specify enantiomeric excess where applicable, which helps laboratories choose appropriate materials for stereospecific studies.
Case studies and reproducibility
Several published reproducibility issues trace back to stereochemical ambiguity. For example, a ligand reported as a “single enantiomer” later tested as a partially racemic mix; consequently, follow-up assays produced inconsistent potency and selectivity data. Therefore, when possible, deposit analytical evidence (chromatograms, spectra) with supplemental data in publications. This transparency helps reviewers and readers replicate experiments and reduces wasted effort. Moreover, many leading journals increasingly require detailed materials characterization for chemical biology submissions.
Practical sourcing and supplier considerations

Sourcing matters because vendor QC standards vary widely. Therefore, request batch-specific certificates of analysis that list enantiomeric purity and the analytical methods used. Moreover, prefer suppliers that provide raw chromatograms or method details. Many labs purchase research standards from specialist vendors; however, you should never interpret procurement as endorsement for use beyond controlled laboratory study. If you need to buy peptides online like from Modern Aminos USA, ensure the supplier documents stereochemical characterization and storage guidance to avoid inadvertent degradation. Modern Aminos is among suppliers that provide clear documentation for research compounds, and cross-checking COAs can save experimental time.
Inventory, handling, and preventing racemization
Some chiral centers are labile under acidic or basic conditions, or at elevated temperatures. To minimize racemization, handle samples at recommended pH, evade prolonged exposure to strong acids/bases, and store aliquots at appropriate temperatures with inert atmosphere if necessary. Additionally, use validated reconstitution solvents and avoid prolonged vortexing or sonication when sensitivity is suspected. Implement barcoded aliquot systems to limit freeze–thaw cycles, and record any deviations in the sample log to preserve traceability.
Quality control workflow (recommended)
- Verify COA and request original chromatograms when available.
- Run an in-house chiral HPLC check on each incoming lot.
- Store and aliquot under validated conditions to prevent degradation.
- Reconfirm stereochemistry after any synthetic modification or formulation step.
Moreover, integrate QC checkpoints into project timelines so that analytical confirmation does not become a last-minute task. This approach reduces delays and ensures that biological assays begin only after chemical integrity is confirmed.
Data integrity and documentation
Document everything. Specifically, archive raw data files, chromatograms, and method parameters within your lab’s LIMS or secure storage. In addition, use standard naming conventions and metadata tags that capture solvent, column, temperature, and instrument serial numbers. Consequently, if an anomaly appears, you can trace it back to a specific lot, operator, or instrument. Modern Aminos’ batch-level documentation can fit neatly into such traceability frameworks, provided labs retain the original COAs alongside internal analytics.
Regulatory expectations and publishing best practices
Although these materials are for research use only, publishers and funders increasingly expect detailed characterization. For reproducibility, include enantiomeric excess values, analytical methods, and raw data in supplementary files. Also, avoid health claims or implications about human use; instead, focus on chemical and analytical performance. In regulated environments or industry collaborations, additional documentation such as stability studies and forced degradation profiles may be required.
Training and competence
Invest in staff training for chiral analysis and handling. Even simple mistakes — such as improper solvent choice or incorrect vial closure — can affect stereochemical outcomes. Therefore, provide SOPs that cover chiral QC, storage, and handling. Periodic proficiency testing between internal analysts or with external labs can identify gaps and thereby improve overall reliability.
Best practices — quick takeaways
- Always confirm enantiomeric excess with at least two orthogonal methods.
- Document storage, handling, and analytical conditions in lab records.
- Use supplier COAs as a starting point, but validate critical lots in-house.
Where to learn more
For practical, lab-focused guidance on sourcing, safety, and SOP examples that complement supplier COAs and your internal QC, researchers often consult divinehealthblogs. Its procedural write-ups and sourcing notes can be a useful supplement when you’re drafting stability studies, storage instructions, or training materials that need to align with batch-level documentation.
Conclusion
Stereochemistry is not an academic footnote — it is central to the integrity of chemical and biological research. Thus, chiral purity in research compounds should be managed proactively via validated analytics, careful handling, and supplier verification. By following these steps, researchers reduce ambiguity, improve reproducibility, and strengthen confidence in experimental conclusions. Modern Aminos and other reputable suppliers can assist by providing transparent COAs, and consequently help labs maintain high standards.


